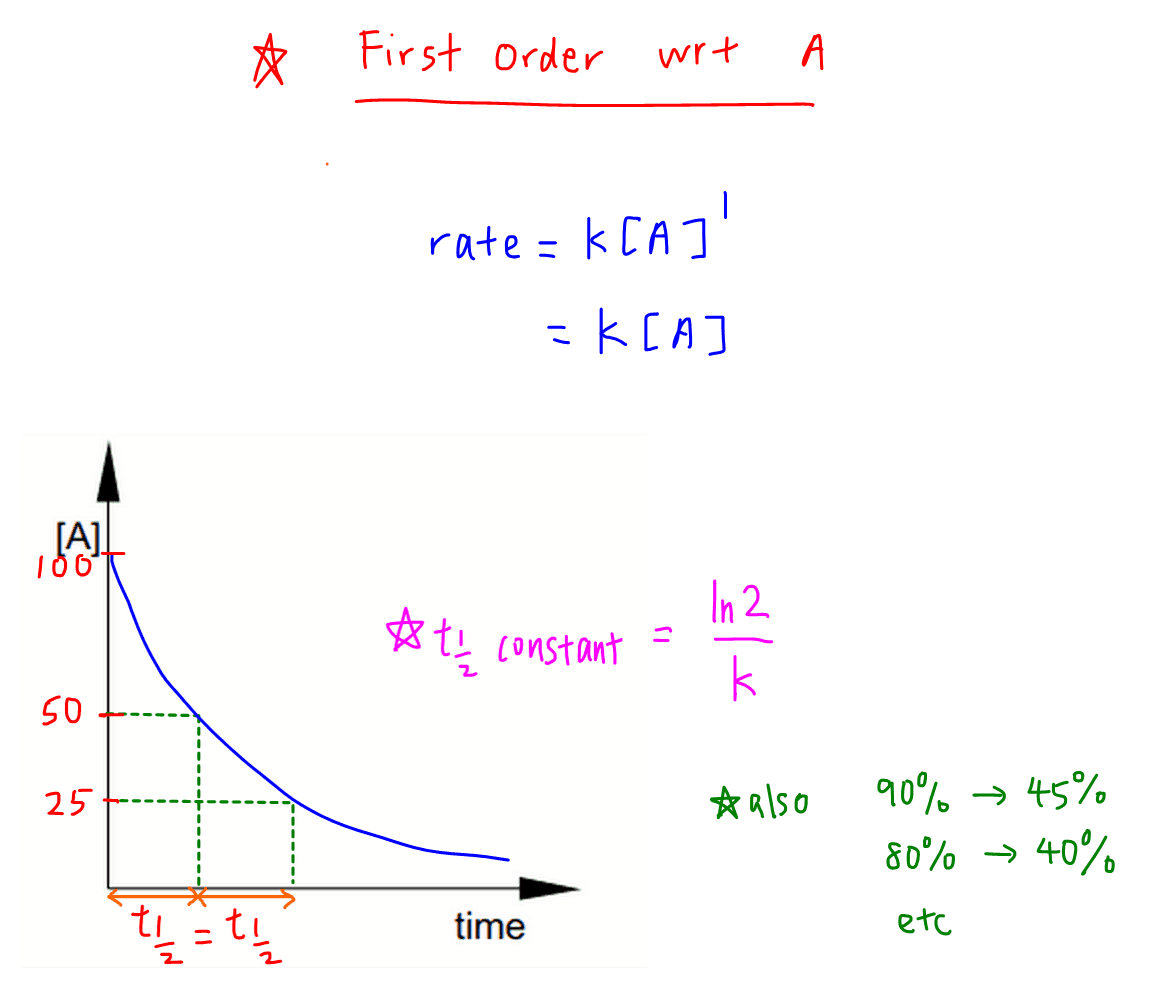
Half Life Equation For First Order Reaction .t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. What is its rate constant?
from mahalast.weebly.com
t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T1 / 2 = 0.693 k. T 1 /2 = 0.693/k.
First order half life equation mahalast
Half Life Equation For First Order Reaction t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T 1 /2 = 0.693/k. What is its rate constant?
From animalia-life.club
Half Life Chemistry Formula Half Life Equation For First Order Reaction What is its rate constant?t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T1 / 2 = 0.693 k. T 1 /2 = 0.693/k. Half Life Equation For First Order Reaction.
From byjus.com
How to calculate the half life of a first order reaction Half Life Equation For First Order Reaction T 1 /2 = 0.693/k. What is its rate constant? T1 / 2 = 0.693 k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. Half Life Equation For First Order Reaction.
From www.toppr.com
The half life of a first order reaction is 480s . Then, the rate Half Life Equation For First Order Reactiont1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. What is its rate constant? T 1 /2 = 0.693/k. T1 / 2 = 0.693 k. Half Life Equation For First Order Reaction.
From www.youtube.com
Determine the halflife of a first order reaction YouTube Half Life Equation For First Order Reaction What is its rate constant? T1 / 2 = 0.693 k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T 1 /2 = 0.693/k. Half Life Equation For First Order Reaction.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For First Order Reaction T1 / 2 = 0.693 k. T 1 /2 = 0.693/k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. What is its rate constant? Half Life Equation For First Order Reaction.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For First Order Reactiont1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T 1 /2 = 0.693/k. T1 / 2 = 0.693 k. What is its rate constant? Half Life Equation For First Order Reaction.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation For First Order Reaction T 1 /2 = 0.693/k. T1 / 2 = 0.693 k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. What is its rate constant? Half Life Equation For First Order Reaction.
From general.chemistrysteps.com
HalfLife of a Reaction Chemistry Steps Half Life Equation For First Order Reaction T1 / 2 = 0.693 k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T 1 /2 = 0.693/k. What is its rate constant? Half Life Equation For First Order Reaction.
From slidesharenow.blogspot.com
Half Life Equation First Order slideshare Half Life Equation For First Order Reaction T1 / 2 = 0.693 k. What is its rate constant?t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T 1 /2 = 0.693/k. Half Life Equation For First Order Reaction.
From www.youtube.com
Halflife of a firstorder reaction AP Chemistry Khan Half Life Equation For First Order Reaction T1 / 2 = 0.693 k. What is its rate constant? T 1 /2 = 0.693/k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. Half Life Equation For First Order Reaction.
From www.youtube.com
14.4 Half Life for 1st Order Reactions YouTube Half Life Equation For First Order Reactiont1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. What is its rate constant? T1 / 2 = 0.693 k. T 1 /2 = 0.693/k. Half Life Equation For First Order Reaction.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation For First Order Reaction T1 / 2 = 0.693 k. What is its rate constant? T 1 /2 = 0.693/k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. Half Life Equation For First Order Reaction.
From haipernews.com
How To Calculate Half Life Chemistry Pdf Haiper Half Life Equation For First Order Reactiont1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T1 / 2 = 0.693 k. T 1 /2 = 0.693/k. What is its rate constant? Half Life Equation For First Order Reaction.
From www.chegg.com
Solved Halflife equation for firstorder reactions 0.693 Half Life Equation For First Order Reaction What is its rate constant? T 1 /2 = 0.693/k.t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T1 / 2 = 0.693 k. Half Life Equation For First Order Reaction.
From slidesharenow.blogspot.com
Half Life Equation First Order slideshare Half Life Equation For First Order Reactiont1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T 1 /2 = 0.693/k. T1 / 2 = 0.693 k. What is its rate constant? Half Life Equation For First Order Reaction.
From lavelle.chem.ucla.edu
halflife CHEMISTRY COMMUNITY Half Life Equation For First Order Reaction T 1 /2 = 0.693/k. What is its rate constant?t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. T1 / 2 = 0.693 k. Half Life Equation For First Order Reaction.
From www.showme.com
Finding halflife for a first order reaction Science, Chemistry Half Life Equation For First Order Reactiont1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. What is its rate constant? T 1 /2 = 0.693/k. T1 / 2 = 0.693 k. Half Life Equation For First Order Reaction.
From www.chegg.com
Solved Halflife equation for firstorder reactions t/2 = Half Life Equation For First Order Reaction T 1 /2 = 0.693/k. T1 / 2 = 0.693 k. What is its rate constant?t1 / 2 = ln [a]0 1 2[a]0 × 1 k = ln2 × 1 k = 0.693 × 1 k. Half Life Equation For First Order Reaction.